Abstract
Background: Patients with beta-thalassemia may need to start receiving regular red blood cell transfusions as early as infancy. In the absence of iron chelation therapy, frequent transfusions cause iron to accumulate in the body, which can lead to morbidity, organ damage, and death. However, in very young children, current practice is to delay chelation therapy until receipt of 10-20 transfusions, or until the patient's serum ferritin (SF) level has reached 1000 μg/L, due to concerns over excessive iron depletion observed with the parenteral iron chelator deferoxamine. Unfortunately, this delay may increase the risk of iron accumulation in endocrine glands such as the pancreas, pituitary, and thyroid, where toxicities could manifest later in life. The START study (NCT03591575) evaluated the safety and efficacy of the oral iron chelator deferiprone (DFP) in children with transfusion-dependent beta-thalassemia who did not yet meet the criteria for starting chelation therapy as per standard practice.
Methods: Infants and children who had started on a regular blood transfusion regimen, received a minimum of 2 transfusions, and whose SF level was between 200 and 600 μg/L were randomly assigned 1:1 to receive either (DFP) oral solution or a matching volume of placebo until their SF levels exceeded 1000 μg/L at 2 consecutive visits or they completed 12 months of therapy, whichever occurred first. The dosage of DFP was initiated at 25 mg/kg/day and increased to 50 mg/kg/day after 2 weeks; based on iron load, the dosage was then increased to 75 mg/kg/day for some patients. All adverse events (AEs) were reported and assessed for causality. Efficacy was assessed by monthly SF measurements to monitor iron load, with the primary efficacy endpoint being the percentage of patients in each group whose SF was still below the 1000 μg/L threshold. Once withdrawn, the patients were to receive standard chelation therapy, as recommended by their primary care physician.
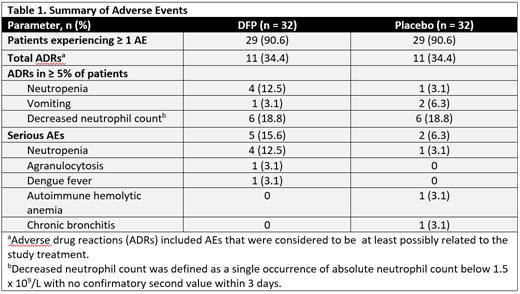
Results: The study enrolled 64 patients; 32 in each group. The mean (SD) age was 3.03 (2.42) years in the DFP group and 2.63 (1.70) years in the placebo group; the participants were 62.5% and 65.6% male in the DFP and placebo groups, respectively. There were no statistical group differences in the baseline demographics. The primary reason for withdrawal was SF levels that exceeded 1000 μg/L at 2 consecutive visits, which occurred in 25% of patients receiving DFP compared with 63% of patients receiving placebo (P=0.0051). After completing 12 months of treatment, 65.6% of patients receiving DFP had a SF level <1000 mg/L compared with 37.5% receiving placebo (P=0.0446). The percentage of patients who reached the 1000 mg/L SF threshold increased more rapidly in the placebo group compared with the DFP group, and the difference in rates between the 2 groups was significant (P=0.0407). A summary of adverse events (AEs) is shown in Table 1. There were no significant group differences in the number of overall AEs (P=1.0000), serious AEs (P=0.4258), or the number of AEs that were considered to be at least possibly related to the study treatment (P=1.0000). Two patients receiving DFP withdrew from the study due to AEs: 1 patient experienced agranulocytosis and 1 patient experienced neutropenia of moderate severity and both patients recovered.
Conclusions:
Initiation of DFP chelation therapy at a lower threshold of SF values than currently recommended was safe and efficacious in preventing iron overload in most transfusion-dependent children. After 12 months of treatment, the number of patients below the SF threshold was significantly higher in the DFP group compared with the placebo group. Moreover, there was a significant difference in the number of patients who withdrew due to elevated SF levels in the placebo group (62.5%) compared with the DFP group (25%). The safety and tolerability profile of DFP administered for up to 12 months in infants and young children was comparable to the profile established in older age groups and there were no instances of iron depletion.
Disclosures:
This study was sponsored by Chiesi Global Rare Diseases, and medical writing support was provided by Kelsey Hodge-Hanson, PhD, of Oxford PharmaGenesis Inc., Newtown, PA, and funded by Chiesi.
Fradette: Chiesi Canada Corp: Current Employment. Lee: Chiesi Canada Corp: Current Employment. Temin: Chiesi Canada Corp: Current Employment. Tricta: Chiesi Canada Corp: Current Employment. Rozova: Chiesi Canada Corp: Current Employment. Hamdy: Novartis: Honoraria; Roche: Honoraria; NovoNordisk: Honoraria; Bayer: Honoraria; Takeda: Honoraria; Amgen: Honoraria; ApoPharma: Honoraria.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal